Preparing Solutions Activity
Modified from Helms, Biology in the Laboratory
Solutions are homogeneous mixtures of atoms, molecules, or ions of two or more different substances. The dissolved substance is called the solute, the dissolving medium is called the solvent. Making solutions of known composition is one of the technically important aspects of science, since most chemical reactions occur in solution. To study these reactions it is necessary to know how to make solutions properly. We are now going to learn the types of solutions in common use, their properties and their uses. These types of solutions include: molar, molal, normal, percent by weight, and percent by volume solutions. We will also learn how to make dilutions.
Molar Solutions
A solution is 1 Molar (M) when it contains 1 mole of solute in a liter of solution. One mole of a substance is the gram molecular weight or the molecular weight in grams of that substance. One mole of a substance contains Avogadro's number of molecules of that substance. For instance, the molecular weight of HCl is 36.46 (mass of each of the elements from the periodic table added together). Thus, a mole of HCl has a mass of 36.46g. To prepare a 1M solution of NaCl, you determine the molecular weight for NaCl by adding the masses of Na and Cl (23 + 35.4) to get 58.4. Add 58.5 grams of NaCl to a 1000ml volumetric flask. Add water to the 1000ml mark. Notice that a molar solution is defined in moles of substance per liter of solution not per liter of solvent. Thus, the total of water and solute which make up the solution must equal 1000ml. If you added 58.5 grams of NaCl to 1000ml of water you would end up with more than 1000 ml of solution. An easy way to determine the number of grams for a solution of a certain molarity is as follows: grams required=molecular weight (in g) X volume (in L) X molarity
Explain how would you prepare:
1) a 0.03 M solution of NaCl
2) a 2M solution of NaOH
3) a 1 X 104 M solution of Na2CO3
Molal Solutions
A solution is 1 molal (m) when it contains one mole of solute plus 1000g (1kg) of solvent. To make up a molal solution, add your solute to 1000ml or 1000g of water since water has a density of 1g/ml. Also, be care to distinguish between the symbols for molar (M) and molal (m). To illustrate this, prepare the following solutions and record the final volume of each solution.
Explain how to and prepare 100ml of molal solution for each of the following:
4) a 1 m solution of NaCl Record final volume.
5) a 0.1 m solution of NaCl Record final volume.
6) a 0.5 m solution of sucrose (C12H22O11) Record final volume.
7) a 0.05 m solution of sucrose (C12H22O11) Record final volume.
8) Compare the volumes of each of the solutions above with the same molar solution of each.
Normal Solutions
Many solutions react with each other, for example, acids with bases. When dealing with solutions of this type it is convenient to use measure of solution that have equivalent numbers off reacting particles. Solutions based on this criteria are referred to as Normal solutions. The molecular weight of a substance determined from the periodic table is the mass of 1 mole (6.02 X 1023) of the substances. If the substance does not separate into particles when put into the solvent then there is no different between Molar solutions and Normal solutions. But if they separate into ions in solution, Normal solutions take into account the number of reacting particles. In neutralization reactions, you need to determine the the number of (H+) ions and equivalent (OH-) ions in the solution. To prepare a 1 N solution of HCl, you would use 36.4 grams of HCl in a liter of solution because HCl only has one H+. To prepare a 1N solution of H2SO4, however, you have to divide the molecular weight by two (98/2=49 grams in a liter of solution) since there will be two H+ ions in each molecule.
Explain how you would prepare:
9) a 1 N solution of NaOH
10) a 1 N solution of Ca(OH)2
Percentage Solutions By Volume
Concentrations of a solution of two liquids are often prepared as percent by volume, rather than weight per unit volume, because volumes are easy to measure. This is commonly done when preparing dilute solution of alcohol. This can be done volumetrically by using a graduated cylinder. To calculate the volumes needed to make a percent volume solution use this equation: C1V1=C2V2 where C1 is the percent concentration of the stock solution, and V1 is the volume needed of the stock solution, C2 is the percent concentration of the desired solution and V2 is the volume desired of the new solution. You can use molar concentrations in place of percent concentrations in the equation as well. To prepare 100ml of a 70% solution of alcohol from a 95% stock solution, the volume you would need would be. (.95)X=(0.70)(100) X=73.68 ml of stock solution and then fill to 100ml with water to get 100ml of 70% solution.
Explain how you would prepare:
11) 200 ml of a 30% solution of alcohol using a 95% stock solution?
12) 250 ml of 2 M solution of HCl using a 12.6 M solution of HCl?
Percentage Solution By Weight
A solution is 1% by weight (w/v) when it contains 1 g of solute per 100ml of solution.
Explain how you would prepare:
13) a 4% solution of NaCl
14) 0.1% solution of gelatin
15) 10 ml of a 6% NaOH solution
Osmolar Solution
The osmolar concentration of a solute is the molar concentration multiplied by the total number of particles produced per molecule in solution. For glucose, C6H12O6, which does not dissociate in solution, a 1 M solution is also 1 osmolar, but for NaCl which dissociates or separates into two particles (Na+ and Cl-) a 1 M solution is 2 osmolar. Two solutions producing the same osmotic effect are said to be isoosmolar (isotonic). The osmolar concentration of plasma which bathes the red blood cells of the body si 0.308. A glucose solution which is 0.308 M is also 0.308 osmolar and is, therefore, isotonic to plasma. Cells bathed in either plasma or or 0.308 M glucose will not show any shrinking or swelling (osmotic effects).
16) A solution of NaCl that is isotonic to plasma will be _______M.
17) A solution of CaCl2 that is isotonic to plasma will be _______M.
Preparing Serial Dilutions
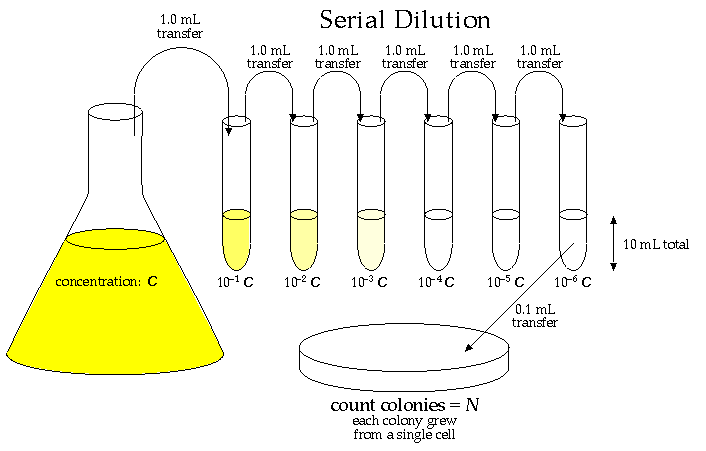
Often the volume you wish to measure is too small to measure accurately with a pipette. For instance suppose you wanted 100 of a 1 X 10-6 M solution of NaOH. To make this solution you would have to weigh out 0.000040g of NaOH. This is beyond the capacity of most balances. You can however, make this solution with precision by using the technique of serial dilution. The method of serial dilution is also widely used by microbiologists to estimate numbers of viable bacteria in various fluids. In some cases the technique of serial dilution is used for preparation of media used for cell culture, the media usually containing trace amounts of essential amino acids and vitamins. Suppose you were making some culture media for an experiment and your recipe called for µg/liter amounts of each constituent. Since your balance is only accurate to the second decimal place (hundredths) you decided to make stock solutions, containing 1 gram/liter, of each component. This means that your stock solution is 106 more concentrated than you wish it to be. Since it would be almost impossible to make 1:1,000,000 dilution accurately, you decide to use the technique of serial dilutions to the the solutions. To do this you use 6 test tubes (since it is 1:106) and fill them each with 9 ml of water or buffer. To the first tube add 1 ml of the concentrated stock solution causing the volume in the first test tube to increase to 10 ml and a 1:10 dilution. Mix the content of the tube well and then transfer 1 ml of solution from the first tube to the second tube making a 1:100ml dilution of the stock solution. This procedure is repeated six times until the original stock solution is diluted 1:1,000,000.

Explain how you would prepare:
18) 10 ml of 1X10-7 M solution of NaOH.
The dilution above is often called a 1 to 10 dilution or a tenfold dilution. Note that you do not always have to use 1 ml and 9ml. You could make a tenfold dilution by mixing 0.5 ml of sample with 4.5 ml of diluent just as easily. If you needed to mae a hundredfold dilution you could mix 0.05ml with 4.95 ml diluent. If you wish to make a 1 to 2 you would add 1 ml of the stock solution to be diluted to 1ml of the diluent to give a final volume of 2ml.
Explain how you would prepare:
19) 75 ml of a particular solution which required a twofold dilution of the material you had on hand.
20) a 1:5 dilution of a broth solution containing 1000 bacteria/ml. How many bacteria would be present in your final solution made by this fivefold dilution.
Now that you have had practice with the various types of solutions...
Explain how you would make 100ml of each of the following solutions.
21) a 0.2 M solution of KH2PO4.
22) a 0.01 N solution of Ca(OH)2.
23) a 0.1 M NaOH, 0.1 M KH2PO4 solution.
24) a 20% solution of red dye by using 50% red dye stock solution.
25) a 1% (w/v) solution of NaHCO3.
26) a 0.1m solution of KH2PO4
27) a 1 X 10-4 solution of green culture medium using the stock solution provided.
Then actually make #23, #24 and #27 and get your results checked off by your teacher.
28) Get initialed for #23. What is the pH of this solution? What is its H+ concentration?
29) Get initialed for #24.
30) Get initialed for #27.