
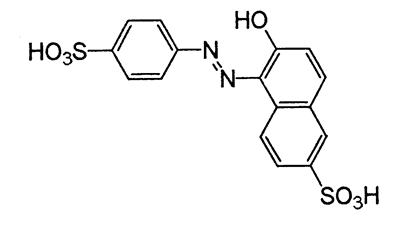
 Yellow
6
Yellow
6Investigation 5: How do you Separate Molecule that are Attracted to one Another? (modified from AP Guided Inquiry Experiments)
Challenge:
Design the "greenest", most effective method to separate the dye molecules in the Kool-Aid mixture using chromatography.
Pre-lab Questions:
1. How can molecules attract each other when they are in a mixture? Predict how ethanol would interact with the dye molecules. Draw a picture illustrating the interactions between the components of the mixture and the solvent, ethanol.
2. What does the Rf value describe on a microscopic level? Why is this important?
3. If the molecule had a very high affinity for the stationary phase, how would this affect the Rf value? Explain.
4. What role does the mobile phase play in the distance a molecule travels in chromatography? What does the mobile phase describe?
5. If you combined a polar solvent with a molecule that has a carbonyl group (carbon with a double-bonded oxygen), would it have a high or low Rf value? Justify your answer with what you understand of intermolecular forces.
Things to Consider when Designing you Procedure:
You will also need to evaluate it in terms of “greenness.” In modern chemistry, chemists use principles of green chemistry to evaluate the solvents that are used in a chemical process for their level of toxicity to humans and the environment. Solvents are also evaluated in terms of their life cycle or how long the molecule remains in the environment and if the molecule breaks down to become more benign or more toxic. The overall focus of green chemistry is to be more efficient in chemical production, producing less waste, using fewer toxic molecules, and producing waste that biodegrades and does not pose a risk to the environment. See Tables 1 and 2 in the GSK solvent selection guide, available at http://pubs.rsc.org/en/content/articlelanding/2011/gc/c0gc00918k under “supplementary information,” for an evaluation of solvents based on these guidelines.
Drawings of molecules in mixture:

 Yellow
6
Yellow
6
Drawings of molecules in solvents:

You should test more than one solvent to determine the most effective.
Post-lab Analysis Questions:
1. Why did you select the solvents that you tested? Did you select the most effective solvents at separating the molecules?
2. What explanations can you provide that caused your separation of the three molecules? How was the choice of the solvent connected to the separation process?
3. What part of the chromatography setup did the molecules interact with, stationary or mobile phase? How would you explain this interaction using intermolecular forces?
4. Draw a picture of how the chromatography worked. Explain your picture using the following terms: stationary phase, mobile phase, and intermolecular forces.
5. Evaluate which solvent is the one with the best “green chemistry” rating (using the reference in the background section). What intermolecular forces would this solvent form with the three molecules in the mixture?
6. Which molecule spent the most time in the stationary phase and why?
7. Calculate the Rf values for each chromatography trial that you completed. Then determine average Rf values for each test condition.
8. Calculate standard deviation for each molecule in each test condition.
Conclusion:
As usual using blue lab format sheet.